Visitors recently descended upon Vegas for PACK EXPO (Sep 29-Oct 1), the largest tradeshow dedicated to packaging and processing. Hosting more than 2,300 exhibitors, PACK EXPO is produced by The Association for Packaging and Processing Technologies (PMMI). Among those in attendance, Allpax was on hand as part of the expansive, industry-spanning ProMach Metroplex. From … [Read More...] about PACK EXPO 2025 Promotes Sustainability, Automation, and Innovation
Main Content

The Importance of the Control System Provider in Thermal Processing
The 1970s brought the introduction of automated industrial controls to the consumer packaged goods manufacturing industry. As the technology advanced, the long-established method of generating manual paper-based process records continued on. Commercial sterilization technology began leaping forward in the 1980s with the introduction of the programmable logic controller (PLC) to the food and beverage manufacturing […]
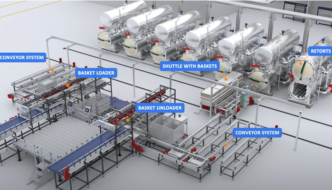
Advancing Sustainable Practices in Retort Systems for RTD Beverage Production
Across manufacturing operations, companies are embracing new approaches to sustainability that balance operational efficiency with responsible resource use. One area … [Read More...] about Advancing Sustainable Practices in Retort Systems for RTD Beverage Production
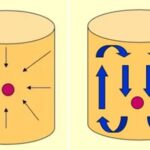
Retort Temperature Distribution and Container Heat Penetration Testing
The thermal process known as commercial sterilization consists of the application of a lethal amount of high heat to effectively kill spoilage and pathogenic organisms such as bacteria and molds. One of the most resilient of these organisms is Clostridium botulinum bacteria. The non-vegetative spore of this particular pathogen is very resistant to high heat. […]

THE VACUUM-COOLED PRODUCT COOKER
A retort that cooks product and also brings the product down to refrigerated temperatures without mechanical refrigeration When large volumes of a food ingredient are processed in a prepared foods manufacturing line, it is almost always desirable to bring the product up to cooking temperature and back down to the desired mixing or blending temperature […]
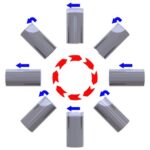
Retort Agitation Methods, Applications and Benefits
One of the benefits of retort processing, also known as commercial sterilization, is that it provides a food or beverage manufacturer the ability to produce a product that is “shelf stable” at room temperature for extended periods of time. The process usually occurs at temperatures ranging from 240°F/115°C to 270°F/132°C. These temperatures exceed that of […]

